FROM LATTICE TO UNIT CELL
![]() S1 lattice
S1 lattice

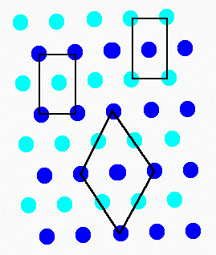
If we start with the lattice, we see that there is often more than one reasonable choice of repeat unit (or unit cell).
In lattice S1, we have shown several. Although there are rules
for selecting the "best" unit cell, for our purposes they are unimportant.
Note that each unit cell can be stacked without rotation to fill the surface
and form the lattice.

Click
here to go to the next page.
Structure of Crystals
Crystal Lattices
Unit Cells
From Unit Cell to lattice
![]() From
Lattice to Unit Cell
From
Lattice to Unit Cell![]()
Stoichiometry
Packing & Geometry
Simple Cubic Metals
Close Packed Structures
Body Centered Cubic
Cesium Chloride
Sodium Chloride
Rhenium Oxide
Niobium Oxide
Except as otherwise noted, all images, movies and
VRMLs are owned and copyright ![]() by
by
Barbara L. Sauls and Frederick C. Sauls 2000.
Contact the owners for individual permission to
use. blsauls@kings.edu